The development safety update report (DSUR) is a harmonized, safety document covering the safety summary of medicinal products under development (including marketed drugs that are under further study) among the International Council for Harmonisation (ICH) regions (replacing US IND Annual Report and EU Annual Safety Report). The guideline for the same is in ICH E2F.
DSUR focuses (before and after marketing authorization) on
- Clinical trials of investigational drugs (including vaccines and biologics), and
- Other findings that impact the safety and welfare of clinical trial subjects (e.g., non-clinical studies, observational studies, relevant manufacturing changes, lack of efficacy, literature data)
- Providing information on comparators where relevant to the safety of trial participants
- Safety findings from clinical trials conducted by co-development partners
- Non-interventional trials/ compassionate use
The objectives of a DSUR are
- Annual review and evaluation of safety information, in accord with previous knowledge of the product’s safety
- Description of new issues that may impact the overall program or specific clinical trials
- Summarization of current understanding and management of known and potential safety risks (identified and potential) to exposed patients
- Examine changes in the product´s safety profile
- Provide an update on the status of the clinical development program and results
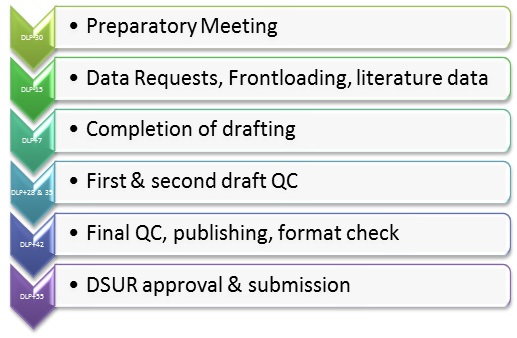
A single DSUR including safety data from all clinical trials conducted with the drug should be prepared for an investigational drug: investigational drug- all indications, all dosage forms, all formulations, and all intended populations. The data lock point (DLP) of the DSUR is generally the Development International Birth Date (DIBD). When clinical trials continue after receiving market approval, both DSUR and PBRER are needed separately. The dates of a DSUR submission can be synchronised by preparing a DSUR based on the International Birth Date (IBD). Then the DLP of the DSUR, the DIBD, is aligned to the IBD. However, the first DSUR period should not be longer than one year (updated annually). The DSUR is always submitted on a yearly basis. The IBD date cannot be changed.
The core contents of a DSUR are
- Introduction
- Worldwide Marketing Approval Status
- Actions Taken in the Reporting Period for Safety Reasons
- Changes to Reference Safety Information
- Inventory of Clinical Trials Ongoing and Completed
- Estimated Cumulative Exposure
- Data in Line Listings and Summary Tabulations
- Significant Findings from Clinical Trials
- Safety Findings from Non-interventional Studies
- Other Clinical Trial/Study Safety Information
- Safety Findings from Marketing Experience
- Non-clinical Data
- Literature
- Other DSURs
- Lack of Efficacy
- Region-specific Information
- Late-Breaking Information
- Overall Safety Assessments
- Summary of Important Risks
- Conclusions
The Reference Safety Information to be considered is the Investigators Brochure in effect at the start of the reporting period. Line listing, tabulations, and narratives may include unblinded clinical trial data; however, sponsor should not unblind data for the specific purpose of developing a DSUR. DSUR is submitted to regulatory authorities within 60 days from the DIBD. Executive Summary (plus line listing of SADRs) may be submitted to ethics committee and investigators, if required. A perfect DSUR is the one which contains clear, concise, consistent information of the ongoing safety profile and action taken to minimize the risk to patient’s participation in the ongoing trials.