The term “pharmacovigilance” includes the science and activities involved in the detection, assessment, understanding, and prevention of adverse effects of medicines. The definition and scope of pharmacovigilance has evolved and extended to monitoring throughout the lifecycle of a medicine (i.e. from the drug development stage to the post-marketing stage) and risk minimization activities to reduce the risk and increase the benefit. There is now added focus on the risk-benefit analysis of the medicines as well.
Thus, “pharmacovigilance writing” or “safety writing” continues to play a crucial role in the pharmacovigilance process. This requires the presence of specialised writers for successful navigation through the various sets of technical requirements. These safety documents needs to be written within the regulatory framework that governs the content of the document, and be compliant with the current guidelines, which can be modified to meet specific company and product requirements, so as to meet the stringent timelines with good quality.
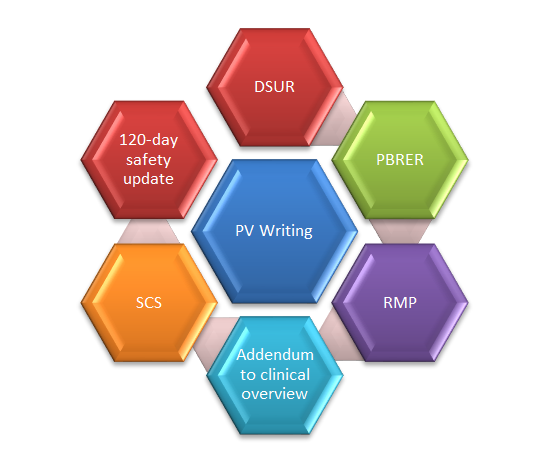
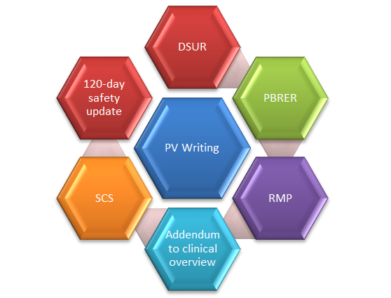
Few safety documents that are written in the clinical development phase are (DSUR, Annual reports, and Narratives); in preparation of marketing authorisation (Summary of Clinical Safety, Integrated Summary of Safety, Clinical Overviews, Risk Management Plans); post-marketing phase (PBRERs, 120-Day Safety Update Reports etc.) Since many safety documents continue to be updated throughout the life cycle, the need for pharmacovigilance medical writing expertise remains constant.
*DSUR= Development Safety Update Report, PBRER=Periodic Benefit Risk Evaluation Report, PV=Pharmacovigilance, RMP=Risk Management Plan, SCS=Summary of Clinical Safety
The pharmacovigilance medical writer should have extensive knowledge and expertise of the specific pharmacovigilance related guidelines (general and country specific requirements), content of the document (sections and what goes into each section), style of writing and presenting, data sources required. These skills along with efficient project management ensure that the presentation of patient safety, the drug’s benefit/ risk profile and the company’s risk management assessments to regulatory authorities are clear, consistent, and transparent across the whole set of pharmacovigilance documents.
As a specialist service provider in this area, we continue to provide content writing guidance on report structure and project management expertise to our clients and cater to different requirements for all types of safety reports.
Please contact us for a confidential discussion of your project writing needs.